Press Releases
Bioxodes announces the completion of the active phase of its First-in-Human study performed in healthy adult volunteers with Ir-CPI
Marche-en-Famenne, Belgium, April 29th, 2021
Bioxodes, a clinical stage biotechnology company announces today the completion of the active phase of its First-in-Human (FIH) study with Ir-CPI dedicated to the prevention of thrombosis. This FIH was a double blind, placebo controlled, single ascending dose trial performed in healthy adult male volunteers with Ir-CPI.
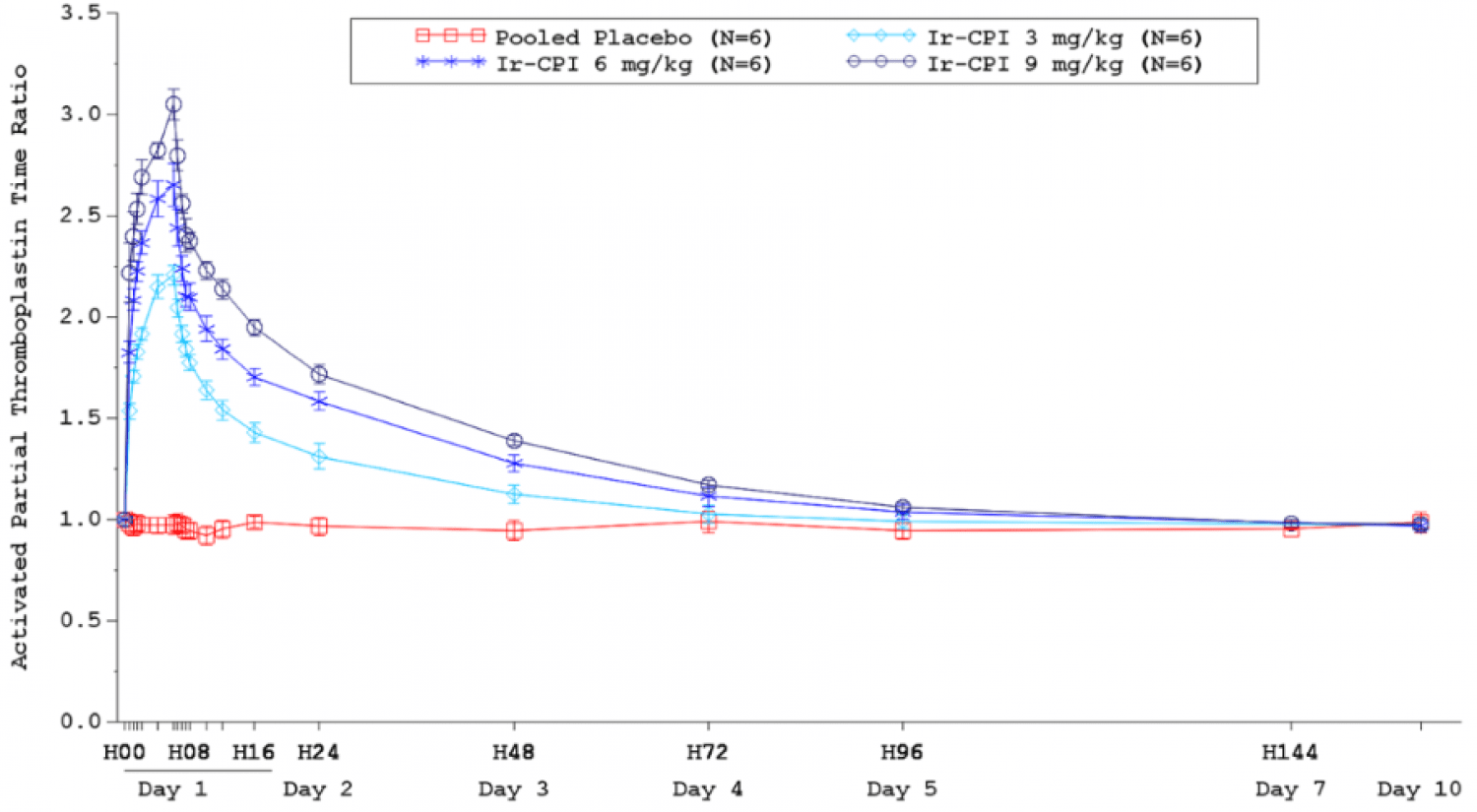
“We are excited by these data from the First-in-Human study. Our drug candidate, Ir-CPI, had a favorable safety profile at each dose level, as well as promising correlation between plasma concentrations and biological activity in healthy volunteers. The results show a dose-dependent prolongation of the activated partial thromboplastin time (aPTT) and inhibition of Factor XI (FXI) and Factor XII (FXII) activity, specific parameters assessing the activity of Ir-CPI on the intrinsic coagulation cascade. These data set a strong foundation for moving into the next stage of development and Phase II trials” declared Edmond Godfroid, Chief Executive Officer of Bioxodes.
Thirty-two (32) healthy adult male volunteers were enrolled in this trial with a ratio 6-active and 2-placebo per dose level. A total of four dose levels of Ir-CPI ranging from 1.5 mg/kg, 3 mg/kg, 6 mg/kg, up to 9 mg/kg were tested and administered intravenously for a 6-h period of infusion.
The primary objectives of the study were to evaluate the safety and tolerability of Ir-CPI. Secondary study objectives included pharmacokinetic (PK) and pharmacodynamic (PD) assessment of Ir-CPI, i.e. the effect on prolongation of the aPTT coagulation parameter and on the inhibition of FXI and FXII activity.
Summary of the Data
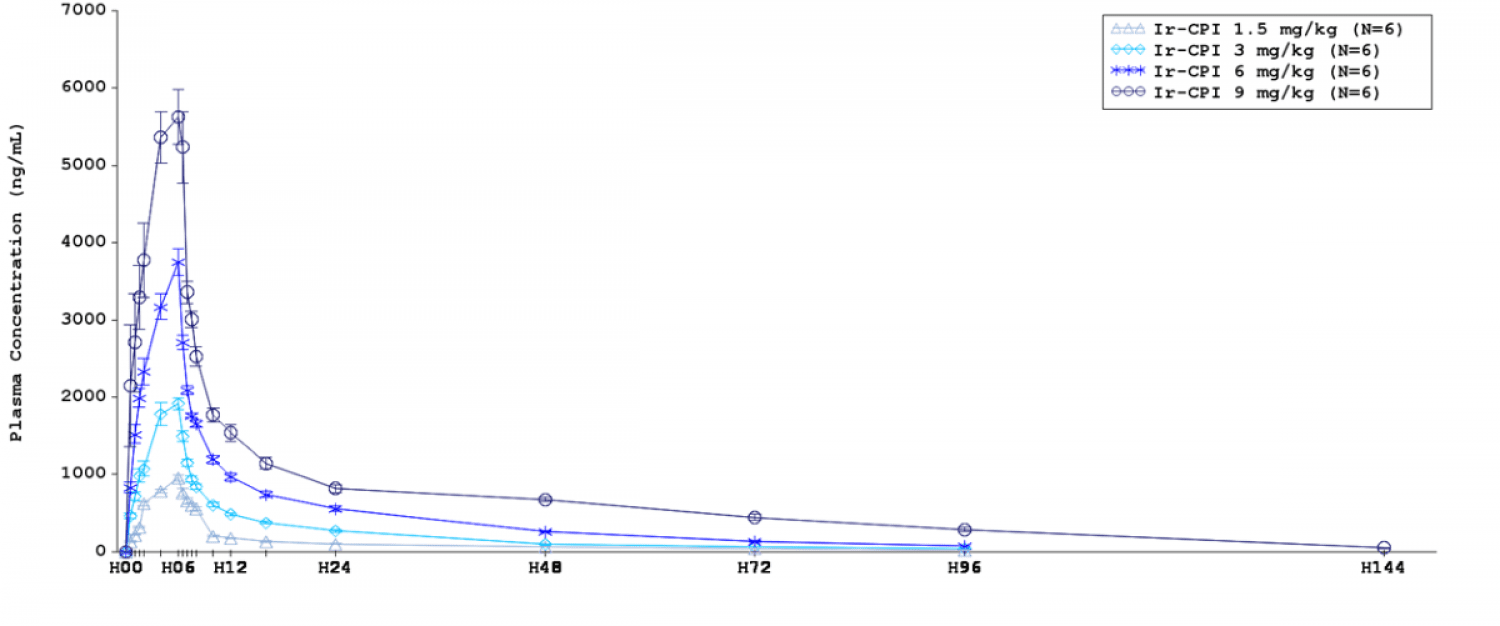
Ir-CPI was well tolerated with no dose-limiting toxicities; there were no safety issues, no related serious adverse events nor adverse events of specific interest, i.e. adverse events related to bleeding. Plasma exposure during infusion (AUC0-6h) and mean peak plasma concentrations increased dose-proportionally. PK profile of escalating single IV infusion doses of Ir-CPI in healthy male participants was characterized by maximum mean plasma concentrations observed at the end of infusion (i.e. 6 hours) (Figure 1). Exposure-dependent changes were observed in the PD parameters: aPTT (Figure 2) and FXI and FXII procoagulant activities. There was a correlation between Ir-CPI plasma concentrations and biological activity.
About Bioxodes
Bioxodes is dedicated to the development of novel therapeutics in the prevention and treatment of thrombotic and inflammatory diseases. Since its setup in 2013, Bioxodes has been developing its lead program based on the Ir-CPI molecule. Ir-CPI is the first candidate in a novel class of therapeutic solutions for patients with thrombo-inflammatory diseases and potentially the first antithrombotic injectable molecule with limited risk of bleeding. The company has so far successfully raised €21.25M from Belgian investment funds and Business Angels, including €7.5M of non-dilutive funding from the Walloon Region. Bioxodes owns issued and pending patents related to Ir-CPI in the EU and US.
About Ir-CPI
Clinical practice shows that the main unmet need in the field of thrombosis prevention is the availability of an antithrombotic therapy without bleeding risk. Thanks to its unique mode of action targeting both coagulation Factors XIIa and XIa (1), Ir-CPI is a promising alternative to current anticoagulants since deficiencies in these factors protect against thrombosis without causing spontaneous bleeding. Indeed, Ir-CPI has been proven to be as efficient as unfractionated heparin, the current gold standard, but devoid of bleeding risk, as shown in preclinical large animal models including a cardiopulmonary bypass (2).
References
1. Demoulin, S., E. Godfroid, and C. Hermans, Dual inhibition of factor XIIa and factor XIa as a therapeutic approach for safe thromboprotection. J Thromb Haemost, 2021. 19(2): p. 323-329.
2. Pireaux, V., et al., Anticoagulation With an Inhibitor of Factors XIa and XIIa During Cardiopulmonary Bypass. J Am Coll Cardiol, 2019. 74(17): p. 2178-2189.