Valérie PireauxA, Tassignon JoëlA, Stéphanie DemoulinA, Sandrine DerochetteA, Nicolas BorensteinB, Angélique EnteB, Laurence FietteB, Jonathan DouxfilsC, Patrizio LancellottiD, Michel GuyauxA, Edmond GodfroidA
- A Bioxodes, Marche-en-Famenne, Belgium
- B IMMR, Paris, France
- C QUALIblood S.A., Namur, Belgium
- D GIGA Cardiovascular Sciences, Department of Cardiology, University Hospital of Liège, CHU Sart Tilman, Liège, Belgium
Documents
Background
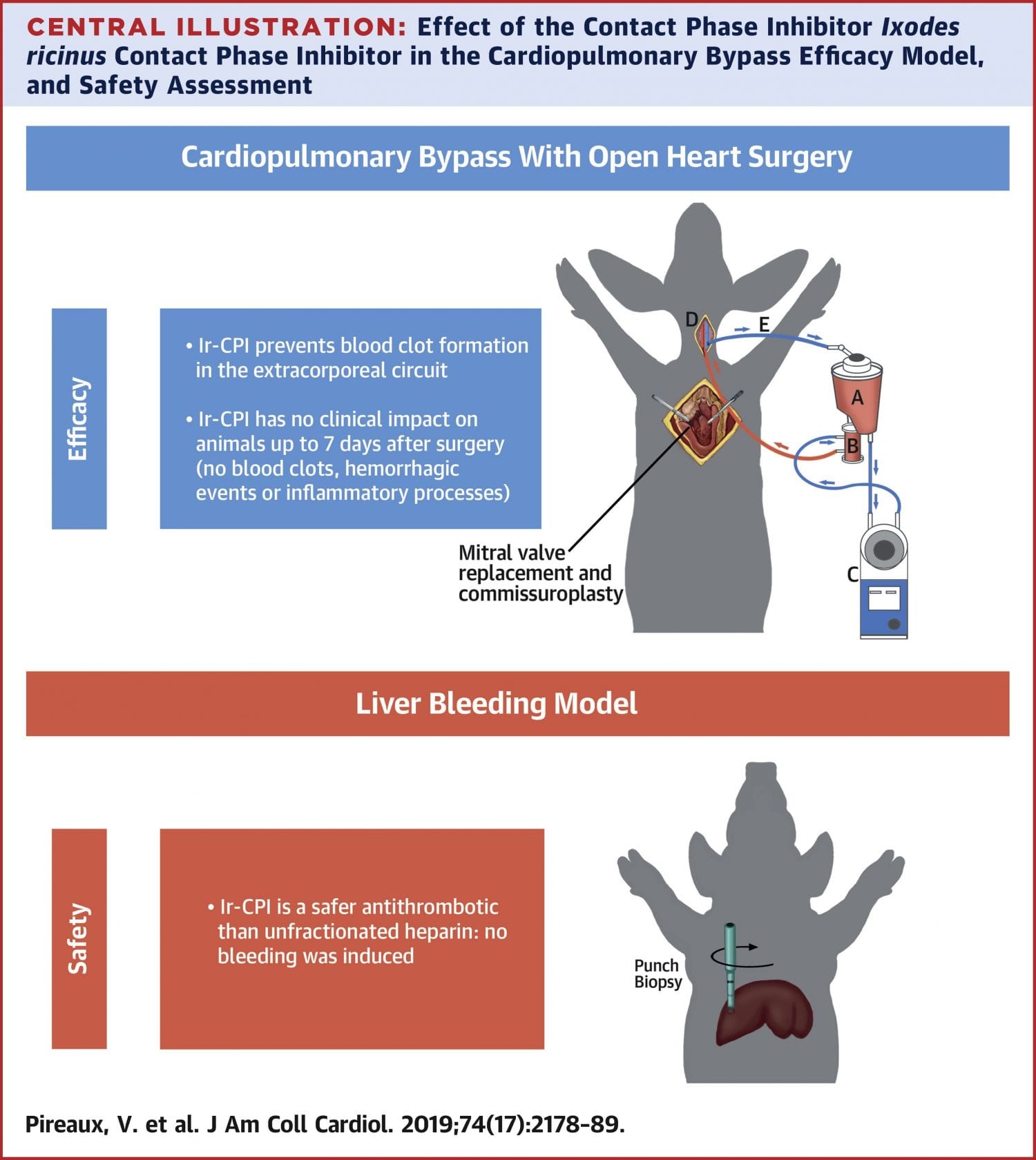
Exposure of blood to polyanionic artificial surfaces, for example, during cardiopulmonary bypass (CPB), induces a highly procoagulant condition requiring strong anticoagulation. Unfractionated heparin (UFH) is currently used during CPB but can lead to serious bleeding complications or development of a hypercoagulable state culminating in life-threatening thrombosis, highlighting the need for safer antithrombotics. Ixodes ricinus contact phase inhibitor (Ir-CPI) is a protein expressed by I. ricinus ticks, which specifically inhibits both factors XIIa and XIa, 2 factors contributing to thrombotic disease while playing a limited role in hemostasis.
Objectives
This study assessed the antithrombotic activity of Ir-CPI in animal contact phase-initiated thrombosis models, including CPB. The safety of Ir-CPI also was evaluated.
Methods
The authors evaluated the antithrombotic activity of Ir-CPI by using in vitro catheter-induced clotting assays and rabbit experimental models of catheter occlusion and arteriovenous shunt. During CPB with cardiac surgery in sheep, the clinical applicability of Ir-CPI was investigated and its efficacy compared to that of UFH using an uncoated system suitable for adult therapy. Taking advantage of the similar hemostatic properties of pigs and humans, the authors performed pig liver bleeding assays to evaluate the safety of Ir-CPI.
Results
Ir-CPI prevented clotting in catheter and arteriovenous shunt rabbit models. During CPB, Ir-CPI was as efficient as UFH in preventing clot formation within the extracorporeal circuit and maintained physiological parameters during and post-surgery. Unlike UFH, Ir-CPI did not promote bleeding.
Conclusions
Preclinical animal models used in this study showed that Ir-CPI is an effective and safe antithrombotic agent that provides a clinically relevant approach to thrombosis prevention in bypass systems, including highly thrombogenic CPB.